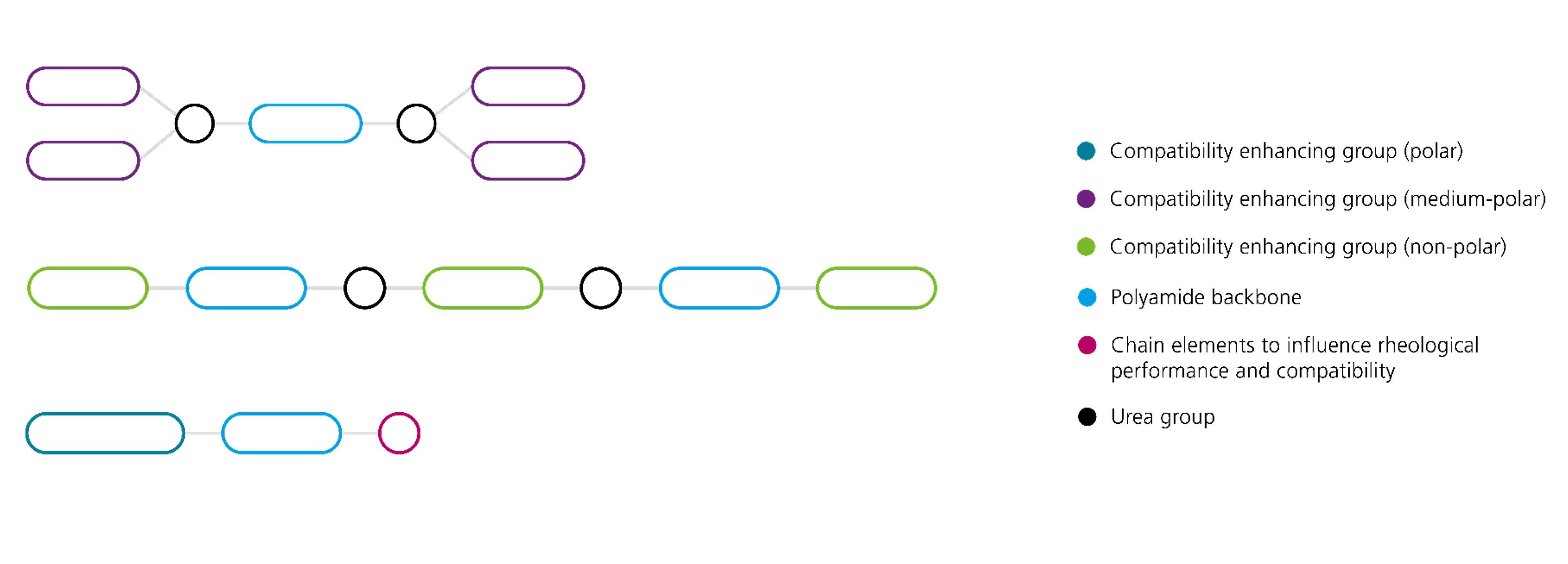In the product class of liquid polyamides, the advantages of urea chemistry and its easy handling are combined with those of diamides and their pseudoplastic flow behavior. The combination of these features is unique and corresponding products are suitable for the entire polarity range (from non-polar to polar) depending on the chemical modification of the product. Products are available for solvent-borne and solvent-free as well as for aqueous systems.
Polyamides are supplied as additives in dissolved form. When the low viscous products are incorporated by laminar stirring, a three-dimensional structure is built up depending on the polarity of the system. At ideal polarity and compatibility, the structure is built up effectively and a pseudoplastic rheological effect is created. With reduced compatibility, the effectiveness is lower because ideal structure formation is no longer possible. In many cases, this reduction in performance can be compensated by higher temperature (better solubility), for example during grinding. The pseudoplastic flow behavior generated by polyamides is particularly suitable for increasing viscosity (in-can viscosity) and for achieving higher film thickness during application.
In addition, polyamides generate viscoelastic behavior in the application system and are therefore also used to improve storage stability, anti-settling performance, and to optimize the orientation of effect pigments. Compared to the low-molecular and solid diamides widely used in the market, the liquid polyamides do not require precise temperature control during incorporation. Due to the structure of the polyamides, interactions with pigments and fillers can occur. This usually leads to a stronger rheological effect in pigmented systems but can lead to gloss reduction if pigment stabilization is insufficient.
The active substance of the liquid polyamide-based rheology additives has a polymer structure in which polyamide segments are linked with polarity-controlling units. The functional groups of the polyamide segments allow the formation of a network that is primarily based on the formation of hydrogen bonds and is responsible for the rheological effect. In order to achieve rheological effectiveness in a system, the polyamides must be adjusted with respect to compatibility by incorporating polarity-controlling segments. Furthermore, the rheological effect can be optimized by incorporating additional functional groups into the active substance. If the polarity of the system is not ideal for the complete formation of the supramolecular structure, an increase in temperature (e.g. by grinding) can lead to an improvement in the rheological properties.